摘要 对于局部进展期胃癌或食管胃结合部腺癌进行新辅助治疗已使愈来愈多的患者从中获益,在既往单一化疗的基础上联合靶向药物和免疫治疗有望使更多的患者达到pCR,并提高R 0切除率,降低术后肿瘤复发率,从而进一步改善患者的预后。开展后续临床及基础转化研究有利于筛选进展期胃癌新辅助治疗模式治疗优势人群,为进一步改善进展期胃癌患者的预后提供新的可能性。 (在框内向上滑动手指即可浏览全部参考文献) [1] 苗儒林,李子禹,季加孚. 从中国胃肠肿瘤外科联盟相关数据分析我国早期胃癌诊治现状和发展趋势[J]. 中国实用外科杂志, 2019,39(5):419-423. DOI: 10.19538/j.cjps.issn1005-2208.2019.05.03 . 返回引文位置Google Scholar 百度学术 万方数据 [2] Smyth EC , Nilsson M , Grabsch HI ,et al. Gastric cancer. Lancet[J]. 2020,396(10251):635-648. DOI: 10.1016/S0140-6736(20)31288-5 . 返回引文位置Google Scholar 百度学术 万方数据 [3] Pentheroudakis G . Recent eupdates to the ESMO clinical practice guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer[J]. Ann Oncol, 2019,30(8):1395-1397. DOI: 10.1093/annonc/mdz180 . 返回引文位置Google Scholar 百度学术 万方数据 [4] Wang FH , Shen L , Li J ,et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer[J]. Cancer Commun, 2019,39(1):10. DOI: 10.1186/s40880-019-0349-9 . 返回引文位置Google Scholar 百度学术 万方数据 [5] 田园,杨沛刚,李勇,等. 进展期胃癌新辅助治疗后发生疾病进展患者的临床病理特征及预后分析[J]. 中华普通外科杂志, 2021,36(4):249-253. DOI: 10.3760/cma.j.cn113855-20201213-00929 . 返回引文位置Google Scholar 百度学术 万方数据 [6] Cunningham D , Allum WH , Stenning SP ,et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer[J]. New Engl J Med, 2006,355(1):11-20. DOI: 10.1056/NEJMoa055531 . 返回引文位置Google Scholar 百度学术 万方数据 [7] Ychou M , Boige V , Pignon J P ,et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase Ⅲ trial[J]. J Clin Oncol, 2011,29(13):1715-1721. DOI: 10.1200/JCO.2010.33.0597 . 返回引文位置Google Scholar 百度学术 万方数据 [8] Al-Batran SE , Homann N , Pauligk C ,et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial[J]. Lancet, 2019,393(10184):1948-1957. DOI: 10.1016/S0140-6736(18)32557-1 . 返回引文位置Google Scholar 百度学术 万方数据 [9] Iwasaki Y , TeRAShima M , Mizusawa J ,et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial[J]. Gastric Cancer, 2021,24(2):492-502. DOI: 10.1007/s10120-020-01136-7 . 返回引文位置Google Scholar 百度学术 万方数据 [10] Zhang X , Liang H , Li Z ,et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D 2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial [J]. Lancet, 2021,22(8):1081-1092. DOI: 10.1016/S1470-2045(21)00297-7 . 返回引文位置Google Scholar 百度学术 万方数据 [11] Wang X , Lu C , Wei B ,et al. Perioperative versus adjuvant S-1 plus oxaliplatin chemotherapy for stage Ⅱ/Ⅲ resectable gastric cancer (RESONANCE): a randomized, open-label, phase 3 trial[J]. J Hematol Oncol, 2024,17(1):17. DOI: 10.1186/s13045-024-01536-7 . 返回引文位置Google Scholar 百度学术 万方数据 [12] Kang YK , Yook JH , Park YK ,et al. PRODIGY: a phase Ⅲ study of neoadjuvant docetaxel, oxaliplatin, and S-1plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer[J]. J Clin Oncol, 2021,39(26):2903-2913. DOI: 10.1200/JCO.20.02914 . 返回引文位置Google Scholar 百度学术 万方数据 [13] Jiang Z , Xie Y , Zhang W ,et al. Perioperative chemotherapy with docetaxel plus oxaliplatin and S-1 (DOS) versus oxaliplatin plus S-1 (SOX) for the treatment of locally advanced gastric or gastro-esophageal junction adenocarcinoma (MATCH): an open-label, randomized, phase 2 clinical trial[J]. Gastric Cancer, 2024,27(3):571-579. DOI: 10.1007/s10120-024-01471-z . 返回引文位置Google Scholar 百度学术 万方数据 [14] Bang YJ , Van Cutsem E , Feyereislova A ,et al. TRAStuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2010,376(9742):687-697. DOI: 10.1016/S0140-6736(10)61121-X . 返回引文位置Google Scholar 百度学术 万方数据 [15] Rivera F , Izquierdo-Manuel M , García-Alfonso P ,et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase Ⅱ trial[J]. Eur J Cancer, 2021,145(2):158-167. DOI: 10.1016/j.ejca.2020.12.005 . 返回引文位置Google Scholar 百度学术 万方数据 [16] Wagner AD , Grabsch HI , Mauer M ,et al. EORTC-1203-GITCG-the "INNOVATION"-trial: effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus Pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase Ⅱ-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer Group [J]. BMC Cancer, 2019,19(1):494. DOI: 10.1186/s12885-019-5675-4 . 返回引文位置Google Scholar 百度学术 万方数据 [17] Kataoka K , Tokunaga M , Mizusawa J ,et al. A randomized Phase Ⅱ trial of systemic chemotherapy with and without trastuzumab followed by surgery in HER2-positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301 (Trigger Study)[J]. Japan J Clin Oncol, 2015,45(11):1082-1086. DOI: 10.1093/jjco/hyv134 . 返回引文位置Google Scholar 百度学术 万方数据 [18] Xu RH , Zhang Y , Pan H ,et al. Ef ficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW- Asia): a randomised, multicentre, double-blind, phase 3 trial [J]. Lancet, 2021,6(12):1015-1024. DOI: 10.1016/S2468-1253(21)00313-7 . 返回引文位置Google Scholar 百度学术 万方数据 [19] Ma J , Yao S , Li XS ,et al. Neoadjuvant therapy of DOF regimen plus Bevacizumab can increase surgical resection ratein locally advanced gastric cancer: a randomized, controlled study[J]. Medicine, 2015,94(42):e1489. DOI: 10.1097/MD.0000000000001489 . 返回引文位置Google Scholar 百度学术 万方数据 [20] Cunningham D , Stenning SP , Smyth EC ,et al. Peri-operative chemotherapy with or without Bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial [J]. Lancet, 2017,18(3):357-370. DOI: 10.1016/S1470-2045(17)30043-8 . 返回引文位置Google Scholar 百度学术 万方数据 [21] Zheng Y , Yang X , Yan C ,et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: a single-arm, open-label, phase Ⅱ trial[J]. Eur J Cancer, 2020,130(2):12-19. DOI: 10.1016/j.ejca.2020.02.013 . 返回引文位置Google Scholar 百度学术 万方数据 [22] Janjigian YY , Shitara K , Moehler M ,et al:First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial[J]. Lancet, 2021,398(1):27-40. DOI: 10.1016/s0140-6736(21)00797-2 . 返回引文位置Google Scholar 百度学术 万方数据 [23] Xu J , Jiang H , Pan Y ,et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial[J]. JAMA, 2023,330(21):2064-2074. DOI: 10.1001/jama.2023.19918 . 返回引文位置Google Scholar 百度学术 万方数据 [24] André T , Tougeron D , Piessen G ,et al. Neoadjuvant nivolumab plus Ipilimumab and adjuvant nivolumab in localized de ficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase Ⅱ study [J]. J Clin Oncol, 2023,41(2):255-265. DOI: 10.1200/JCO.22.00686 . 返回引文位置Google Scholar 百度学术 万方数据 [25] Yuan SQ , Nie RC , Jin Y ,et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nature medicine[J]. 2024,30(2):552-559. DOI: 10.1038/s41591-023-02721-w . 返回引文位置Google Scholar 百度学术 万方数据 [26] Shitara K , Rha SY , Wyrwicz LS ,et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysi s of the multicentre, double-blind, randomised phase 3 study [J]. Lancet, 2024,25(2):212-224. DOI: 10.1016/S1470-2045(23)00541-7 . 返回引文位置Google Scholar 百度学术 万方数据 [27] Janjigian YY , Van Cutsem E , Muro K ,et al. MATTERHORN: phase Ⅲ study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal胃癌NAC的发展
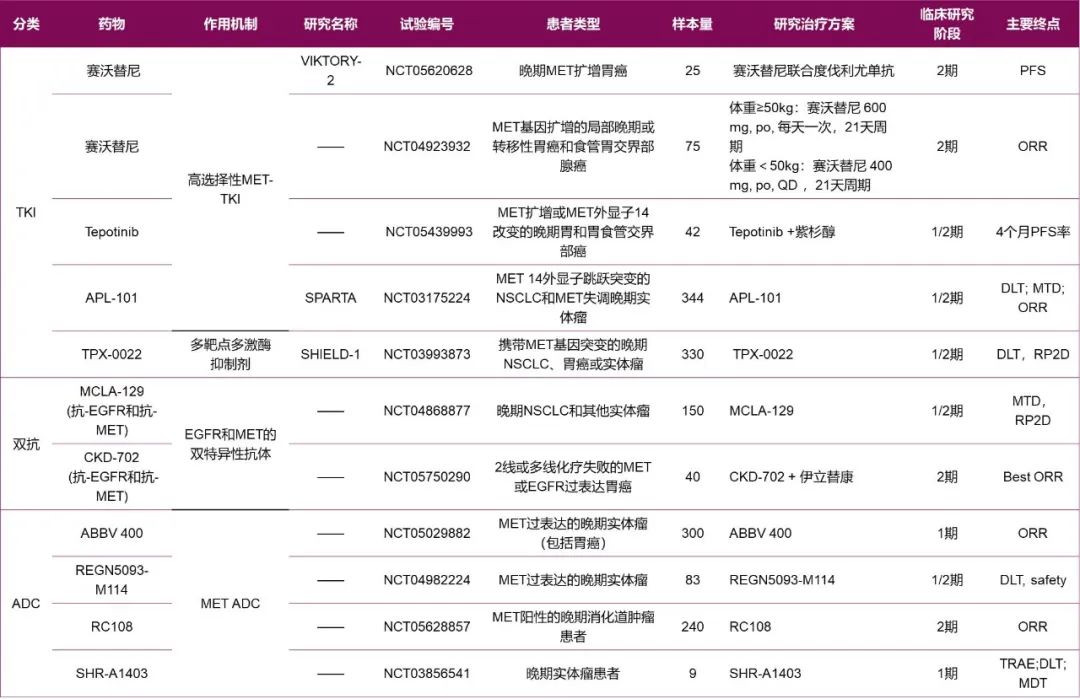
二、靶向药物在胃癌新辅助治疗中的应用
免疫检查点抑制剂在胃癌新辅助治疗中的应用
“靶-免-化”三联疗法在胃癌新辅助治疗中的探索
展望